INTRODUCTION
Avatrombopag (AVA) is a thrombopoietin receptor agonist (TPO-RA), approved for the treatment of immune thrombocytopenia (ITP), as second line in subjects with chronic ITP unresponsive to other treatments. Global response rate of AVA is 69% with a mean duration of 12.4 weeks in its pivotal study 1. The combination of TPO-RA plus immunosuppressive/immunomodulatory drugs could optimize treatment results in ITP, addressing different pathophysiological pathways 2. Among the most widely used immunomodulatory drugs in these circumstances are steroids, azathioprine and mycophenolate. Fostamatinib (FOS) is a splenic tyrosine kinase (SYK) inhibitor. FOS blocks the cascade of signals mediated by SYK after the activation of FcγRs, reducing macrophage activity, proliferation of B lymphocytes and production of antibodies 3.
We present the experience in the combined use of AVA with fostamatinib in non-responders to said TPO-RA, an experience not reported to date.
METHODS
Retrospective, multicenter, international, observational, non-interventional study in patients diagnosed of primary ITP who have received treatment with AVA and FOS in combination between August 2022 and June 2023. We included patients who have not reached platelets >30x10e9/L after at least two weeks of treatment with daily 40mg of AVA, and FOS was prescribed in combination in these circumstances. Epidemiological characteristics, type of ITP, previous treatments received, starting dose, response, concomitant ITP treatment and toxicity are collected. ITP definition and response criteria are based on Provan et al 4: Response (R) as platelets 30-100x10e9/L and complete response (CR) as platelets > 100x10e9/L. Data are described in percentages for the categorical variables and in medians and ranges for the quantitative ones.
RESULTS
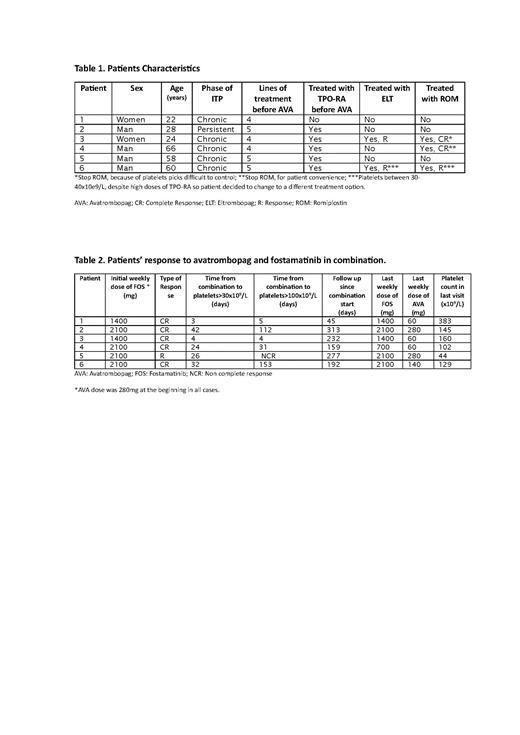
In the period of time evaluated, a total of 55 patients received treatment with AVA in Spain and Norway. The Norwegian data was acquired from the Norwegian ITP registry. In 16 of 55 patients (29%), there was no response after 2 or more weeks with AVA 280 mg weekly. In 6 of these patients, FOS was combined with AVA at a dose of 280mg weekly. Table 1 describes the characteristics of the 6 patients treated with the combination. Median time from initiation of AVA to combination with FOS was 14 days (Range: 14-21 days). The overall response of the combination was 100% (1 R, 5 CR). Median time to R was 25 days and to CR 31 days. Table 2 describes the characteristics of each patient's response. With a median follow-up from the start to last follow up of treatment in combination was 212 days (Range: 45-313 days), there was no relapse. Tapering of AVA and/or FOS was attempted in 5 patients by reducing the dose of FOS in 1 patient and the dose of AVA in 4. In patients 1 and 4, AVA was stopped, but this resulted in drop in the platelets counts. CR was achieved after reintroduction AVA in combination.
With regard to toxicity, in the 6 patients treated with the combination AVA plus FOS, only two adverse events were described, both non-serious. One case of headache encountered with the use of AVA, before the initiation of FOS. The other event, was WHO grade 2 liver toxicity attributed to AVA. Hypertransaminasaemia was resolved after the interruption of avatrombopag for 6 days and reduction of AVA from 140mg to 60mg weekly.
CONCLUSIONS
In subjects with a lack of response to thrombopoietin analogues, the combination with immunosuppressants is an alternative to consider. The combination of avatrombopag and fostamatinib has been shown to be effective and safe, although longer series are needed to support these data.
Bibliography
1. Jurczak W, Chojnowski K, Mayer J, et al. Phase 3 randomised study of avatrombopag, a novel thrombopoietin receptor agonist for the treatment of chronic immune thrombocytopenia. Br J Haematol. 2018;183(3):479-490.
2. Arai Y, Jo T, Matsui H, et al. Comparison of up-front treatments for newly diagnosed immune thrombocytopenia -a systematic review and network meta-analysis. Haematologica. 2018;103(1):163-171.
3. Bussel J, Arnold DM, Grossbard E, et al. Fostamatinib for the treatment of adult persistent and chronic immune thrombocytopenia: Results of two phase 3, randomized, placebo-controlled trials. Am J Hematol. 2018;93(7):921-930.
4. Provan D, Arnold DM, Bussel JB, et al Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780-3817
Disclosures
Ghanima:Kedrion: Consultancy; Argenx: Consultancy, Honoraria; UCB: Consultancy, Honoraria; alpine: Consultancy, Honoraria; Bayer: Consultancy, Honoraria, Research Funding; Grifols: Consultancy, Honoraria; Sobi, Pfizer: Consultancy, Honoraria, Research Funding; BMS: Honoraria, Research Funding; cellphire: Consultancy, Honoraria; hibio: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Amgen: Consultancy, Honoraria.